Our Posters at C Elegans Conference at UCLA
The Elegant Mind Club presents a total of 8 posters this year at
- C. elegans 20th International Meeting
- on June 24-28, 2015 at Pauley Pavilion
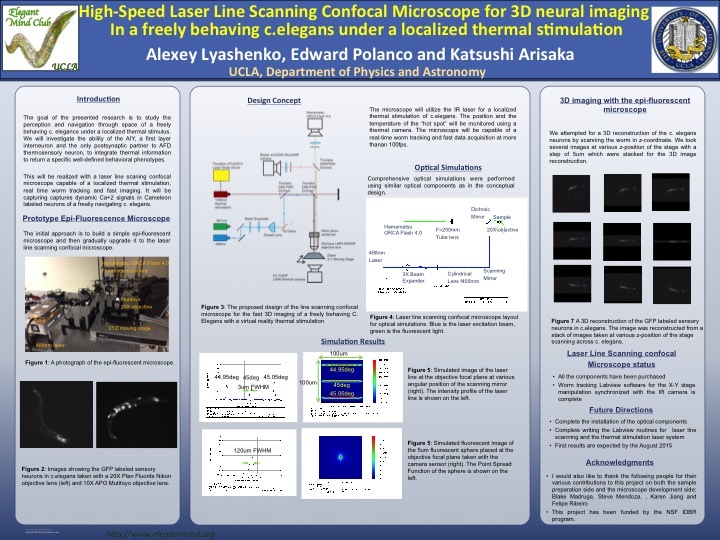
[1] Line scanning fluorescent confocal microscope for a 3D real time neuron imaging of C. elegans
Alexey Lyashenko, Edward Polanco, Katsushi Arisaka
Conventional confocal microscope uses a physical aperture to reduce the amount of out of focus
light to the image sensor. We developed a line scanning confocal microscope that uses a software
controlled rolling shutter on a CMOS camera. This technique allows real time 3d imaging on the
neural network of C. elegans.
Alexey Lyashenko, Edward Polanco, Katsushi Arisaka
Conventional confocal microscope uses a physical aperture to reduce the amount of out of focus
light to the image sensor. We developed a line scanning confocal microscope that uses a software
controlled rolling shutter on a CMOS camera. This technique allows real time 3d imaging on the
neural network of C. elegans.
| alexey_slc_microscope.pdf | |
| File Size: | 1142 kb |
| File Type: | |
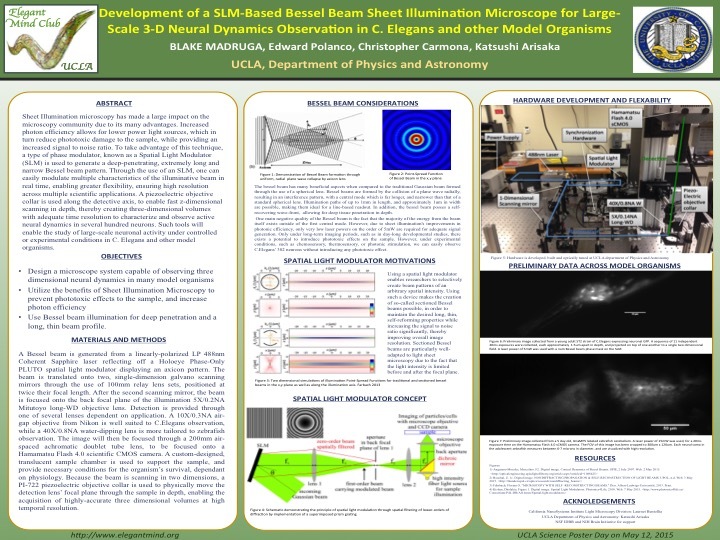
[2] Development of a Bessel Beam Sheet Illumination Microscope for Large-Scale 3-D Neural Dynamics in C. elegans
Blake Madruga, William Chen, Edward Polanco, Christopher Carmona, Steve Mendoza, Paul Chin, Tim Sherry, Katsushi Arisaka
Sheet Illumination has recently amassed a lot of attention as a technique, due to many benefits
over standard microscopy methods. Decreased phototoxicity, increased signal to noise ratio, and
higher photonic efficiency are only a few of the reasons why many researchers are beginning to
answer sensitive scientific questions with sheet illumination microscopy. Due to the unique
properties and biological characteristics of C. elegans, access to sheet illumination microscopy is
limited, costly, and difficult to utilize. The purpose of designing such a device is to bring the
proven benefits of sheet illumination to the C. elegans community, in a intuitive, purposefully-
designed manner. A type of phase modulator, known as a Spatial Light Modulator (SLM) is used
in this case to generate a specific bessel-beam pattern. Through the use of an SLM, one can
easily modulate multiple characteristics of the illuminative beam in real time, enabling great
flexibility, ensuring high resolution across multiple scientific applications. Additionally, the use
of a piezoelectric objective collar allows the rapid capturing of three dimensional volumes,
enabling researchers to examine the dynamics of many neurons in space and time, at sub-micron
axial resolution. Such tools will gain access to the observation of largescale, three dimensional
neuronal activity under controlled or experimental conditions in C. elegans, leading to potential scientific discoveries in the future.
Blake Madruga, William Chen, Edward Polanco, Christopher Carmona, Steve Mendoza, Paul Chin, Tim Sherry, Katsushi Arisaka
Sheet Illumination has recently amassed a lot of attention as a technique, due to many benefits
over standard microscopy methods. Decreased phototoxicity, increased signal to noise ratio, and
higher photonic efficiency are only a few of the reasons why many researchers are beginning to
answer sensitive scientific questions with sheet illumination microscopy. Due to the unique
properties and biological characteristics of C. elegans, access to sheet illumination microscopy is
limited, costly, and difficult to utilize. The purpose of designing such a device is to bring the
proven benefits of sheet illumination to the C. elegans community, in a intuitive, purposefully-
designed manner. A type of phase modulator, known as a Spatial Light Modulator (SLM) is used
in this case to generate a specific bessel-beam pattern. Through the use of an SLM, one can
easily modulate multiple characteristics of the illuminative beam in real time, enabling great
flexibility, ensuring high resolution across multiple scientific applications. Additionally, the use
of a piezoelectric objective collar allows the rapid capturing of three dimensional volumes,
enabling researchers to examine the dynamics of many neurons in space and time, at sub-micron
axial resolution. Such tools will gain access to the observation of largescale, three dimensional
neuronal activity under controlled or experimental conditions in C. elegans, leading to potential scientific discoveries in the future.
| blake_-_slm_bb.pdf | |
| File Size: | 4936 kb |
| File Type: | |
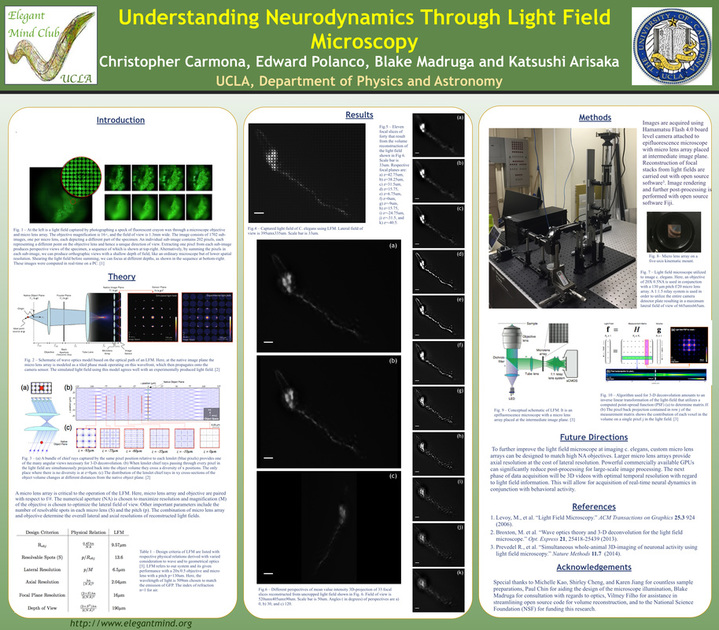
[3] Understanding Neurodynamics Through Light Field Microscopy
Christopher Carmona, Edward Polanco, Blake Madruga, Addam Hammond, Katsushi Arisaka
Light field microscopy is a new technique that allows for quick volumetric imaging of
fluorescent specimens. It utilizes a microlens array (MLA) as a key optical component that
produces a light field and trades spatial resolution against angular resolution or axial resolution.
The MLA is a matrix of lenses with diameters of 130μm that each resolve a visual perspective of
a specimen being imaged at relative distances from the native object plane. As a result, recorded
light-fields can be computationally reconstructed into full volumes. The volume reconstruction is
formulated as an inverse linear transformation that is modeled using wave optics theory. Here, an
epifluorescence light field microscope is designed and configured in order to resolve the neural
activity of C. elegans during real-time inculcated behavior. The theoretical limits of the
microscope’s lateral resolution in relation to optical design choices are discussed and compared
with experimental results. The primary focus of this investigation is the utilization of light field
microscopy in conjunction with computationally intensive image processing methods as a useful
tool for analyzing the behavior and corresponding brain activity of C. elegans. Light field microcopy has the potential to offer real-time 3-D video data of the unrestrained behavior of C. elegans.
Christopher Carmona, Edward Polanco, Blake Madruga, Addam Hammond, Katsushi Arisaka
Light field microscopy is a new technique that allows for quick volumetric imaging of
fluorescent specimens. It utilizes a microlens array (MLA) as a key optical component that
produces a light field and trades spatial resolution against angular resolution or axial resolution.
The MLA is a matrix of lenses with diameters of 130μm that each resolve a visual perspective of
a specimen being imaged at relative distances from the native object plane. As a result, recorded
light-fields can be computationally reconstructed into full volumes. The volume reconstruction is
formulated as an inverse linear transformation that is modeled using wave optics theory. Here, an
epifluorescence light field microscope is designed and configured in order to resolve the neural
activity of C. elegans during real-time inculcated behavior. The theoretical limits of the
microscope’s lateral resolution in relation to optical design choices are discussed and compared
with experimental results. The primary focus of this investigation is the utilization of light field
microscopy in conjunction with computationally intensive image processing methods as a useful
tool for analyzing the behavior and corresponding brain activity of C. elegans. Light field microcopy has the potential to offer real-time 3-D video data of the unrestrained behavior of C. elegans.
| chris_-_lfm.pdf | |
| File Size: | 5044 kb |
| File Type: | |
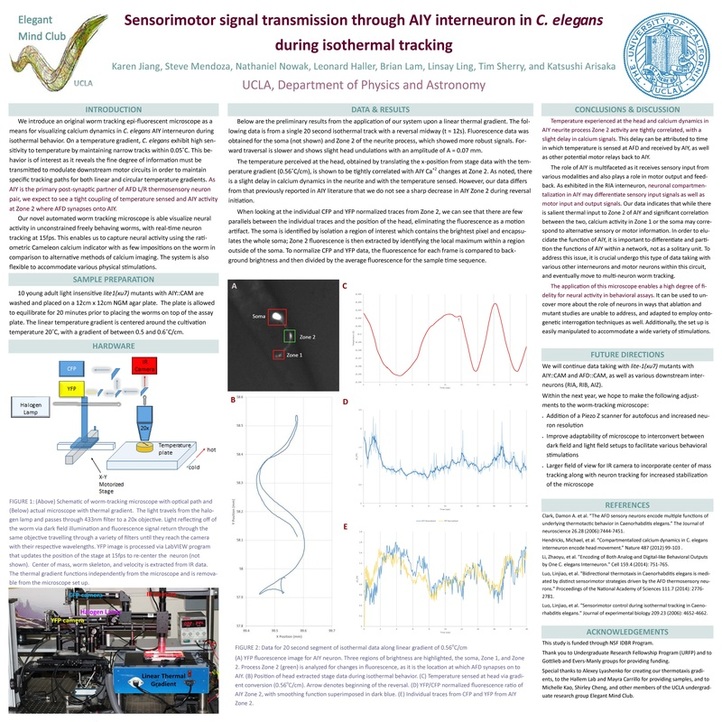
[4] Sensorimotor signal transmission through AIY interneuron in Caenorhabditis elegans during isothermal tracking
Karen Jiang, Steve Mendoza, Nathaniel Nowak, Leonard Haller, Linsay Ling, Tim Sherry, Katsushi Arisaka
Perception and navigation through space require accurate translation and transmission of sensory input to motor output. On a linear temperature gradient, Caenorhabditis elegans demonstrate a
distinct behavioral phenotype in which they frequently travel along isotherms, maintaining
sensitivity within 0.05 °C. This isothermal attractor state is correlated with movement at a
constant and maximal velocity. We investigate how AIY, a first layer interneuron and
postsynaptic partner to AFD thermosensory neuron, is able to integrate thermal information to
return specific well defined behavioral phenotypes. Prior observations of neural activity in vivo
involve partial paralysis or constraint of the worm while stimuli is applied. Other systems
circumvent this limitation by re-centering the stage; this generates an external force during stage
acceleration introducing another stimulus. We overcome these two primary obstacles through the
implementation of a novel automated worm-tracking epi-fluorescent microscope. The three-
camera microscope system mounted on a movable XY stage captures dynamic Ca+2 signals in
Cameleon-labeled neurons while the nematode navigates unconstrained along the temperature
gradient. Implementing this set up, we observed that the greatest temperature difference occurs
between the extremes of the head movement while along isotherms which phase lock with
fluorescence response in AIY. The steady Ca+2 waveform in AIY suppresses reversals and
maintains high speeds to downstream motor circuitry.
Karen Jiang, Steve Mendoza, Nathaniel Nowak, Leonard Haller, Linsay Ling, Tim Sherry, Katsushi Arisaka
Perception and navigation through space require accurate translation and transmission of sensory input to motor output. On a linear temperature gradient, Caenorhabditis elegans demonstrate a
distinct behavioral phenotype in which they frequently travel along isotherms, maintaining
sensitivity within 0.05 °C. This isothermal attractor state is correlated with movement at a
constant and maximal velocity. We investigate how AIY, a first layer interneuron and
postsynaptic partner to AFD thermosensory neuron, is able to integrate thermal information to
return specific well defined behavioral phenotypes. Prior observations of neural activity in vivo
involve partial paralysis or constraint of the worm while stimuli is applied. Other systems
circumvent this limitation by re-centering the stage; this generates an external force during stage
acceleration introducing another stimulus. We overcome these two primary obstacles through the
implementation of a novel automated worm-tracking epi-fluorescent microscope. The three-
camera microscope system mounted on a movable XY stage captures dynamic Ca+2 signals in
Cameleon-labeled neurons while the nematode navigates unconstrained along the temperature
gradient. Implementing this set up, we observed that the greatest temperature difference occurs
between the extremes of the head movement while along isotherms which phase lock with
fluorescence response in AIY. The steady Ca+2 waveform in AIY suppresses reversals and
maintains high speeds to downstream motor circuitry.
| karen-_thermotaxis.pdf | |
| File Size: | 1221 kb |
| File Type: | |
[5] The Elegant Mind Club: Undergraduate Research Club to Explore the Minds of C. elegans
Michelle Kao, Shirley Cheng, Karen Jiang, Taejoon Kim, Nathaniel Nowak, Leonard Haller, Steve Mendoza, De'Marcus Woolfork, Brian Lam, Neha Agarwal, Tim Sherry, Peter Pellionisz, Bobby Vanmali, Linsay Ling, Blake Madruga, GuillaumeTrusz, Addelyn Sagadevan, Sean Atamdede, Amanda Sunyoto, Katsushi Arisaka
For students, the study of model organisms presents an opportunity to learn various general
science and research disciplines; however, each model organism has it’s own set of advantages.
Chiefly, Caenorhabditis elegans are excellent model organisms to study neuroscience and
biophysics due to its availability, tractability, relatively simple nervous system, and it's patently
observable behavior. Here in the Elegant Mind Club at UCLA, we provide undergraduate
students a unique hands-on experience working with C. elegans and a chance to present their
own scientific methods of interest, allowing them to explore the nature of scientific research and
understand the conclusions drawn from their data. In our laboratory, undergraduate students are
entirely responsible for maintaining and culturing the worms as well as building and refining
their experimental systems. Direct involvement with the biological samples teaches students the
discipline of working with chemicals and maintaining sterility. Published papers and online
resources such as WormBook, WormAtlas, and Caenorhabditis Genetics Center provide students
with a source of well-established methods and techniques to serve as a basis for their own
studies. Manual practice in hardware development permits students to personally hone their
experiment to be the most controlled and reproducible systems. As of now, systems for
thermotaxis, electrotaxis, chemotaxis, phototaxis, durotaxis, as well as behavior within a
magnetic field and the absence of stimuli have been reproduced and improved by our members.
Our laboratory begun with a few core members and have expanded to accommodate more than
80 students from different universities over the world and we hope to encourage more students to
approach scientific research with enthusiasm.
Michelle Kao, Shirley Cheng, Karen Jiang, Taejoon Kim, Nathaniel Nowak, Leonard Haller, Steve Mendoza, De'Marcus Woolfork, Brian Lam, Neha Agarwal, Tim Sherry, Peter Pellionisz, Bobby Vanmali, Linsay Ling, Blake Madruga, GuillaumeTrusz, Addelyn Sagadevan, Sean Atamdede, Amanda Sunyoto, Katsushi Arisaka
For students, the study of model organisms presents an opportunity to learn various general
science and research disciplines; however, each model organism has it’s own set of advantages.
Chiefly, Caenorhabditis elegans are excellent model organisms to study neuroscience and
biophysics due to its availability, tractability, relatively simple nervous system, and it's patently
observable behavior. Here in the Elegant Mind Club at UCLA, we provide undergraduate
students a unique hands-on experience working with C. elegans and a chance to present their
own scientific methods of interest, allowing them to explore the nature of scientific research and
understand the conclusions drawn from their data. In our laboratory, undergraduate students are
entirely responsible for maintaining and culturing the worms as well as building and refining
their experimental systems. Direct involvement with the biological samples teaches students the
discipline of working with chemicals and maintaining sterility. Published papers and online
resources such as WormBook, WormAtlas, and Caenorhabditis Genetics Center provide students
with a source of well-established methods and techniques to serve as a basis for their own
studies. Manual practice in hardware development permits students to personally hone their
experiment to be the most controlled and reproducible systems. As of now, systems for
thermotaxis, electrotaxis, chemotaxis, phototaxis, durotaxis, as well as behavior within a
magnetic field and the absence of stimuli have been reproduced and improved by our members.
Our laboratory begun with a few core members and have expanded to accommodate more than
80 students from different universities over the world and we hope to encourage more students to
approach scientific research with enthusiasm.
| michelle_-_elegant_mind_club.pdf | |
| File Size: | 7298 kb |
| File Type: | |
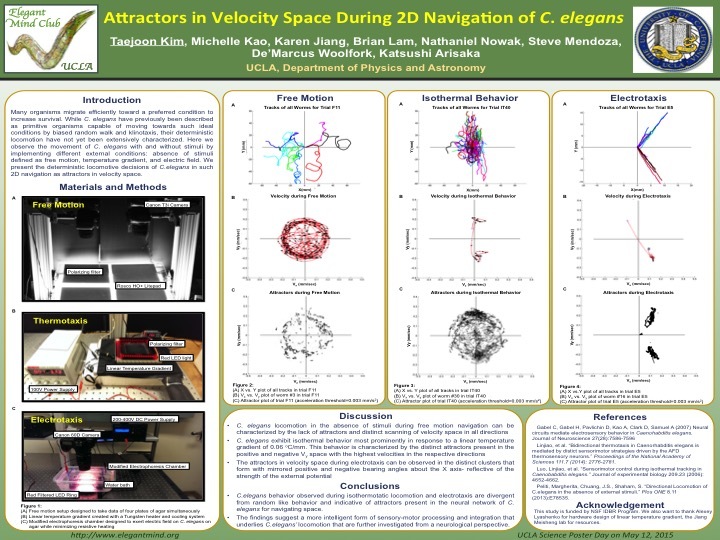
[6] Attractors in Velocity Space During 2D Navigation of C. elegans
Michelle Kao, Taejoon Kim, De'Marcus Woolfork, Brian Lam, Karen Jiang, Nathaniel Nowak, Steve Mendoza, Katsushi Arisaka
Many organisms migrate efficiently towards an attractant or a preferred condition to increase
survival. While C. elegans have been previously described as a primitive organism capable of
moving towards an attractant by biased random walk and klinotaxis, their deterministic
locomotion have not yet been extensively characterized. Here, we observe the movement of C.
elegans with and without external stimuli by implementing different conditions: temperature
gradient, electric field, and free motion. We compare their locomotive decisions defined by their
movements in velocity space, (Vx , Vy). Their movements under each condition are first
recorded and analyzed separately to characterize their locomotive decisions under that specific
condition. The different conditions are then compared within the physical realm of velocity
space. Their deterministic movements can be clearly distinguished in their distinct directionality
and speed based on the plots presented. The absence of stimuli invokes locomotion that is
characteristic of random search. In comparison, the presence of specific external conditions
sanction a more deterministic locomotion, which can be reproduced with a high degree of
accuracy. We present such locomotion as being divergent from random behavior and
indicative of a neural network for C. elegans to navigate space. The findings suggest a more
intelligent form of sensory-motor processing and integration that underlies C. elegans’
locomotion that are further investigated from a neurological perspective.
Michelle Kao, Taejoon Kim, De'Marcus Woolfork, Brian Lam, Karen Jiang, Nathaniel Nowak, Steve Mendoza, Katsushi Arisaka
Many organisms migrate efficiently towards an attractant or a preferred condition to increase
survival. While C. elegans have been previously described as a primitive organism capable of
moving towards an attractant by biased random walk and klinotaxis, their deterministic
locomotion have not yet been extensively characterized. Here, we observe the movement of C.
elegans with and without external stimuli by implementing different conditions: temperature
gradient, electric field, and free motion. We compare their locomotive decisions defined by their
movements in velocity space, (Vx , Vy). Their movements under each condition are first
recorded and analyzed separately to characterize their locomotive decisions under that specific
condition. The different conditions are then compared within the physical realm of velocity
space. Their deterministic movements can be clearly distinguished in their distinct directionality
and speed based on the plots presented. The absence of stimuli invokes locomotion that is
characteristic of random search. In comparison, the presence of specific external conditions
sanction a more deterministic locomotion, which can be reproduced with a high degree of
accuracy. We present such locomotion as being divergent from random behavior and
indicative of a neural network for C. elegans to navigate space. The findings suggest a more
intelligent form of sensory-motor processing and integration that underlies C. elegans’
locomotion that are further investigated from a neurological perspective.
| michelle_-_free_motion.pdf | |
| File Size: | 4281 kb |
| File Type: | |
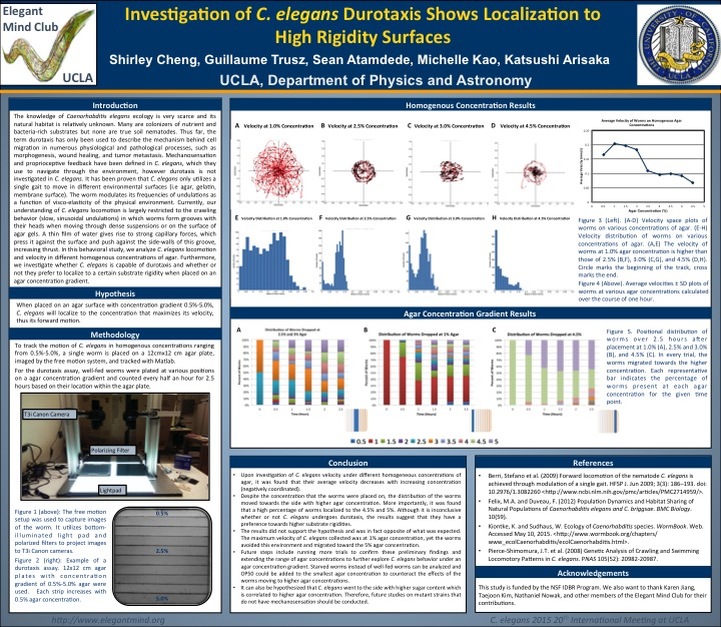
[7] Investigation of C. elegans Durotaxis Shows Localization to High Rigidity Substrates
Shirley Cheng, Guillaume Trusz, Katsushi Arisaka
Mechanosensation is clearly defined in the model organism Caenorhabditis elegans, however
mechanotaxis, more specifically the subset of this behavior termed durotaxis has not been
explored. To date, durotaxis has only been investigated in cell motility in the extracellular matrix
(ECM). C. elegans are nematodes that navigate through the environment by means of
mechanosensation, chemotaxis, thermotaxis, and phototaxis. Therefore they are capable of
receiving and processing sensory input to respond accordingly, similar to certain cell types in the
ECM. It has recently been identified that C. elegans has a single sinusoidal locomotory gait
when moving through different substrate conditions. In lab, culturing C. elegans is maintained on
an agar medium and are limited to a crawling behavior. However, we question if that is the best
condition for culturing C. elegans and if they are capable of distinguishing between different
substrate rigidities. We venture to see if C. elegans is capable of durotaxis after being placed on
an agar gradient. Upon investigation of many combinations of agar concentrations ranging from
0.5%-4%, C. elegans primarily localized to the substrate with higher rigidity. Independent
experiments with homogeneous agar concentrations also show that 3% agar allowed for optimal
motility and velocity of C. elegans. These primitive findings demonstrate that C. elegans can
indeed sense different surface rigidities and provide additional insight to its locomotory
navigation in its natural environment.
Shirley Cheng, Guillaume Trusz, Katsushi Arisaka
Mechanosensation is clearly defined in the model organism Caenorhabditis elegans, however
mechanotaxis, more specifically the subset of this behavior termed durotaxis has not been
explored. To date, durotaxis has only been investigated in cell motility in the extracellular matrix
(ECM). C. elegans are nematodes that navigate through the environment by means of
mechanosensation, chemotaxis, thermotaxis, and phototaxis. Therefore they are capable of
receiving and processing sensory input to respond accordingly, similar to certain cell types in the
ECM. It has recently been identified that C. elegans has a single sinusoidal locomotory gait
when moving through different substrate conditions. In lab, culturing C. elegans is maintained on
an agar medium and are limited to a crawling behavior. However, we question if that is the best
condition for culturing C. elegans and if they are capable of distinguishing between different
substrate rigidities. We venture to see if C. elegans is capable of durotaxis after being placed on
an agar gradient. Upon investigation of many combinations of agar concentrations ranging from
0.5%-4%, C. elegans primarily localized to the substrate with higher rigidity. Independent
experiments with homogeneous agar concentrations also show that 3% agar allowed for optimal
motility and velocity of C. elegans. These primitive findings demonstrate that C. elegans can
indeed sense different surface rigidities and provide additional insight to its locomotory
navigation in its natural environment.
| shirley_-_durotaxis.pdf | |
| File Size: | 14355 kb |
| File Type: | |
[8] A Novel Worm Tracking Calcium Imaging System Utilizing a Mobile Microscope
Steve Mendoza, Tim Sherry, Karen Jiang, Brian Lam, Taejoon Kim, Blake Madruga, Michelle
Kao, Nate Nowak, Katsushi Arisaka
Worm tracking of freely moving worms is essential to study the connection between behavior
and neural activity. However, these microscopes are often limited in their ability to track worms
under various behavioral simulations. We present a fluorescence worm tracking microscope that
has an open geometry and thus can track worms even if the behavioral platform is difficult or
impossible to move via a motorized stage. As opposed to other automated worm tracking
systems, our microscope is fully mobile—where all the optical components are mounted on top
of a motorized xy stage—while the sample stage where C. elegans rests is stationary. This
platform allows for ratiometric calcium imaging while also tracking a dark field worm image for
behavioral analysis, running at 15 frames per second. The current configuration has three
cameras, two for each of the YFP and CFP channels, and a dark field image showing the worm
body, under a 10x magnification; the microscope can also be adjusted to image at 20x. Being
able to track freely moving worms without moving the sample stage, allows our microscope to
perform worm tracking in experimental conditions that similar systems have not been able to
achieve. In addition, since the sample is stationary, we also avoid introducing confounding
effects on the worms due to stage acceleration. To test this novel hardware, we run a thermotaxis
experiment tracking a worm without moving the temperature platform. Our worm was labeled
with AIY::CAM and we find a correlation between the AIY activity and the temperature of the
head location during isothermal behavior. Our methodology could also apply to other behavioral
experiments where an external stimulus would be hard to move via a motorized stage, such as an
electrotaxis experiment.
Steve Mendoza, Tim Sherry, Karen Jiang, Brian Lam, Taejoon Kim, Blake Madruga, Michelle
Kao, Nate Nowak, Katsushi Arisaka
Worm tracking of freely moving worms is essential to study the connection between behavior
and neural activity. However, these microscopes are often limited in their ability to track worms
under various behavioral simulations. We present a fluorescence worm tracking microscope that
has an open geometry and thus can track worms even if the behavioral platform is difficult or
impossible to move via a motorized stage. As opposed to other automated worm tracking
systems, our microscope is fully mobile—where all the optical components are mounted on top
of a motorized xy stage—while the sample stage where C. elegans rests is stationary. This
platform allows for ratiometric calcium imaging while also tracking a dark field worm image for
behavioral analysis, running at 15 frames per second. The current configuration has three
cameras, two for each of the YFP and CFP channels, and a dark field image showing the worm
body, under a 10x magnification; the microscope can also be adjusted to image at 20x. Being
able to track freely moving worms without moving the sample stage, allows our microscope to
perform worm tracking in experimental conditions that similar systems have not been able to
achieve. In addition, since the sample is stationary, we also avoid introducing confounding
effects on the worms due to stage acceleration. To test this novel hardware, we run a thermotaxis
experiment tracking a worm without moving the temperature platform. Our worm was labeled
with AIY::CAM and we find a correlation between the AIY activity and the temperature of the
head location during isothermal behavior. Our methodology could also apply to other behavioral
experiments where an external stimulus would be hard to move via a motorized stage, such as an
electrotaxis experiment.
| steve_-_worm_tracker.pdf | |
| File Size: | 948 kb |
| File Type: | |